Research




We focus on developing innovative strategies to regenerate and rehabilitate functional
muscle tissue following traumatic injuries, specifically volumetric muscle loss (VML) with
or without adjacent bone fracture or peripheral nerve crush.
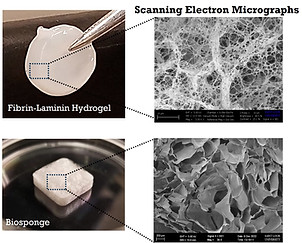
The regenerative approach we are pursuing involves using multifunctional biomaterials
that enhance muscle regeneration and function. These biomaterials are composed of
extracellular matrix proteins that are chemically cross-linked and processed in the form
of biosponges, hydrogels, or micro- or nano-particles for various applications.
Not only do these biomaterials provide mechanical support, but they also deliver growth
factors, pharmaceuticals, stem cells, and exosomes to targeted areas, suppress immune
responses, and act as a substrate for host cell infiltration and proliferation.
We're utilizing mesenchymal stem cells (MSCs) and their secretome
(e.g., growth factors and extracellular vesicles or EVs) to develop a conducive micro-
environment for muscle growth. This strategy involves supporting angiogenesis,
reducing fibrosis, and enhancing immunomodulation at the injury site.
Our approaches for delivering MSCs have included single-cell administration,
utilizing biomaterial-encapsulated cells, and deploying them in spheroidal form.
Current projects use both mouse bone marrow-derived MSCs and human
placenta-derived MSCs.
Our rehabilitative strategy involves neuromuscular electrical stimulation-
mediated strength training to improve muscle mass, size, and torque.
Ongoing research investigates the synergistic effect of combining
regenerative and rehabilitative strategies to optimize their efficacy.
We are proficient in employing small animal models, particularly mice and rats,
to explore skeletal muscle repair and its crosstalk with bone and nerve tissues.
Our predominant research model involves inducing full-thickness VML injuries
in the tibialis anterior or gastrocnemius muscles of rats and mice with or without
adjacent bone fracture or nerve crush. We use these models to determine the
efficacy of our regenerative and rehabilitative approaches through extensive
histological and biomolecular analyses as well as peak isometric torque
measurements. These models are instrumental in offering preclinical insights and a comprehensive
mechanistic understanding of tissue remodeling after injury.
Ongoing efforts are also focused on modulating T-cell activity in the VML-injured
muscle and identifying specific helper T cell subsets (e.g., Th1, Th2, Treg, Th17) that
promote or inhibit satellite cell proliferation/differentiation in vitro or muscle
regeneration in vivo.


Funding Sources (External):
-
National Institute of Health (NIGMS) - 1R15GM129731-01 and 2R15GM129731-02 with 3R15GM129731-02S1
-
National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) - 1R01AR083865-01A1
-
National Institute of Health (NICHD) 1R03HD115731-01
-
National Institute of Health (NIDDK) R15DK132727
-
National Institute of Health (NIGMS) T32GM141602-01A1
-
Department of Defense - Peer Reviewed Orthopedic Research Program (PRORP): W81XWH-18-1-0251 and HT9425-24-1-0592
-
National Science Foundation (I-corps)
-
Center for Defense Medicine at Biogenerator (STL)
-
Joint NC NM4R and AR3T Neuromodulation and Regenerative Rehabilitation Funding Program
Funding Sources (Internal):
-
Institute for Translational Neuroscience (ITN - Saint Louis University)
-
Institute for Drug and Biotherapeutic Innovation Seed Grant (Saint Louis University)
-
Applied Health Sciences Research Grant (Saint Louis University)
-
President's Research Fund (Saint Louis University)
-
Beaumont Faculty Development Fund (Saint Louis University)
-
KEEN Program Transformation Grant (SLU/KEEN)
-
Research Innovation Fund, Start-up Formation Grant, SLU



